Blog
Filter by:

post
Publishing an eCTD Clinical Study Report: ICH E3
Read More
post
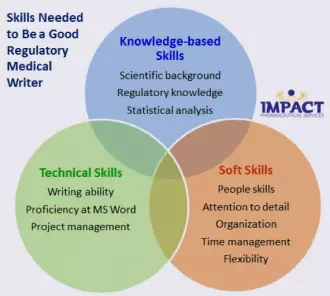
What Skills Do You Need to Be a Good Regulatory Medical Writer?
Read More
post
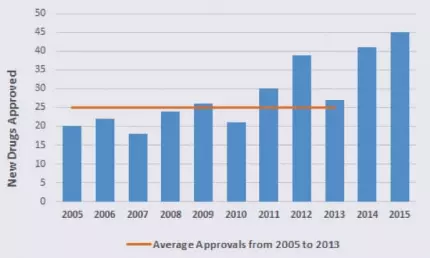
Record Numbers of FDA‑Approved Drugs: Recent Trends
Read More

post
Planning Your NDA or BLA Submission: It’s More Than a Gantt Chart!
Read More
post
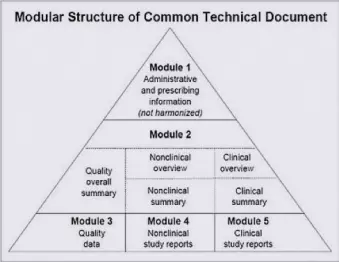
Top Two Things I Learned at DIA RSIDM
Read More
post
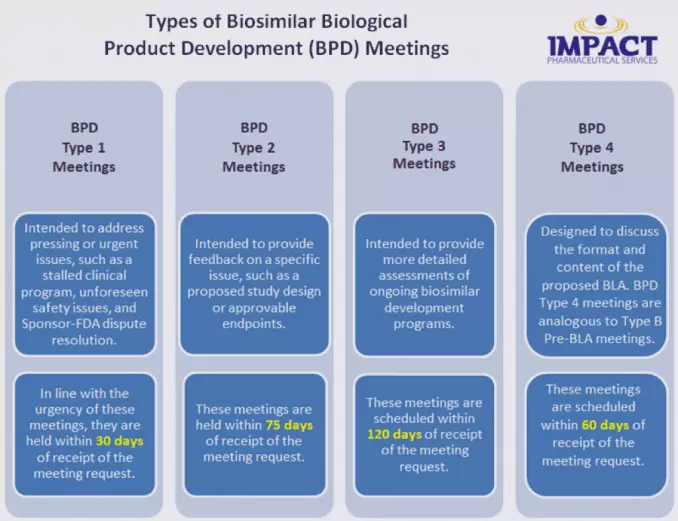
Formal Meetings with FDA for Biosimilar Products
Read More

post
How to Make Publishing Clinical Summaries Easier
Read More
