post
The Importance of Quality in Drug Development with Insights from the FDA Fiscal Year 2023 Report on the State of Pharmaceutical Quality
post
Exploring Risk-Based Approaches to Raw Material Testing in Pharmaceutical Manufacturing
post
Empowering and Encouraging Innovation: The Advantages of the FDA’s Orphan Drug Designation for Drug Companies and Patients
post
Overview of FDA Expedited Development and Approval Programs for Serious Conditions
post
Current Topics in Orphan Drug Development
post
Ready to Submit Your Initial IND?
post
Before you file your IND…
post
The Ins and Outs of INDs
post
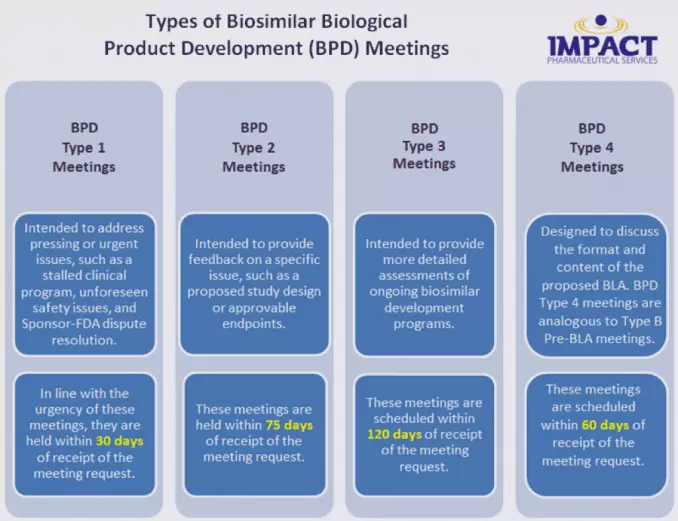
Formal Meetings with FDA for Biosimilar Products