post
Project Orbis: Accelerating Global Access to Innovative Cancer Therapies
post
The Next Generation of Regulatory Submissions: Exploring the Benefits of eCTD 4.0
post
Potency Assurance for Cellular and Gene Therapy Products
post
Conducting Clinical Trials in Australia
post
The Push for Patient-Focused Drug Development
post
Statistics in Harmony: The Role of Estimands in Regulatory Writing
post
Current Topics in Orphan Drug Development
post
Ready to Submit Your Initial IND?
post
Before you file your IND…
post
The Ins and Outs of INDs
post
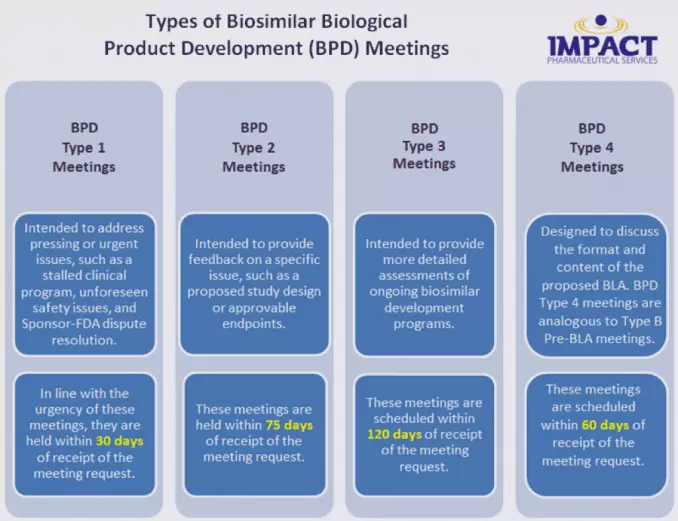
Formal Meetings with FDA for Biosimilar Products
post
Direct-to-Consumer Advertising of Prescription Drugs