Every year, the FDA handles a staggering 1,500 Investigational New Drug (IND) applications, a critical step in bringing pharmaceutical innovations to market. The IND review process is pivotal, ensuring the safety of study subjects and the integrity of clinical trials. To successfully navigate this complex landscape, understanding the FDA’s priorities, common stumbling blocks, and the benefits of pre-IND meetings is essential.
Infographic
Cracking the Code: Syner-G’s Blueprint for Success with Pharmaceutical Stability Studies
Download PDF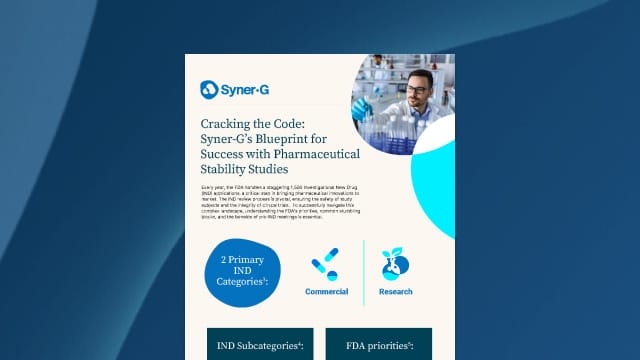
Related Resources
All Resources

post
Oral Drug Product Development of Small Molecules
Read More

post
Quality tools: Resources for Quality Control Specialists in Medical Writing
Read More

white-paper
The New Chemistry, Manufacturing, and Controls (CMC) Regulations in China
Read More

post
Why FDA PreCheck is a game-changer for small & mid-sized pharma — and how these companies should lean in
Read More
Let's move from science to success.
Let’s Talk
