Technical Operations & Delivery Infrastructure
GMP Engineering & Operations

Functional, strategic, and lasting solutions.
Operational readiness has three pillars: people, processes, and technology. True operational readiness ensures these three pillars are ready to perform all their required functions without issues.
At Syner-G, we help our clients achieve operational readiness from a project’s start to ongoing facilities management.
We understand that each site presents its own regulatory, quality, and operational nuances—and we tailor our approach to meet your specific needs.
Challenges we can help solve:
Lack of internal resources
We embed experienced engineers, operators, and project leads who deliver hands-on execution—filling critical capability gaps and accelerating your path to operational scale.
Lack of a coherent, forward-looking development plan
We provide specialized experts and operators with deep life sciences experience, ready to plug in and perform from day one. This allows clients to access expertise without the burden of recruiting, onboarding, and retaining niche talent.
Too many disconnected vendors and handoffs
Our integrated model spans engineering, systems, tech transfer, and GMP operations—giving you a single point of accountability for technical execution. With an operator’s perspective, we help design systems and processes that perform from day one.
Pressure to scale quickly
Speed matters as every day delayed is money lost. We help clients move fast without cutting corners, with patent clocks ticking and commercial timelines looming, we accelerate execution across engineering, validation, and operations—helping you avoid multimillion-dollar delays and hit your launch window.
Missed milestones and unclear readiness plans
We manage complex cross-functional workstreams—from capital projects to operational readiness—so you stay on track, aligned, and audit-ready.
Client Engagements
Technical Projects
Project Delivery
Square Feet Under Management

Trusted partner with thousands of projects completed.
Our Approach
From construction to operation — we make it happen.
Bringing innovative therapies to market demands flawless execution of complex technical projects.
Whether it’s laboratory relocations, facility construction, technology transfers, or startup operations, we lead cross-functional workstreams to ensure your environment, equipment, and teams are fully prepared for GMP compliance and manufacturing success.
We work hand-in-hand with design, construction, engineering, and project and operations teams. Our proven process minimizes disruption, accelerates timelines, and keeps your science and manufacturing teams fully operational throughout the transition.
- Deep Domain & Practical Expertise: engineers, technical project managers, CQV experts, GMP specialists
- Flexible & Scalable Solutions: capital programs, scale-up, automation, validation, tech transfer
- Dedicated, Full-time Support: no third-party contractors, just committed partners
- Regulatory & GMP Excellence: hands-on operational experience in regulated environments

We can help with:
Facility Builds, Upgrades, and Expansions
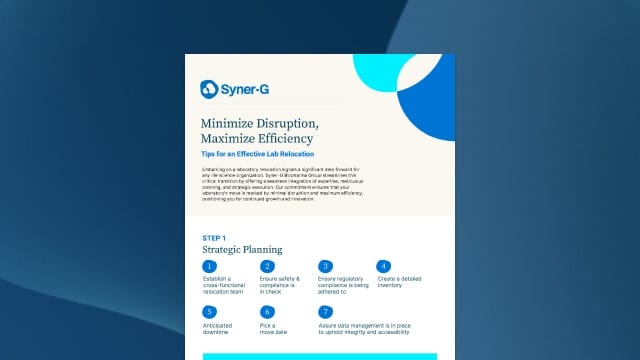
Laboratory and Manufacturing Moves
Commissioning, Qualification, and Validation (CQV)
Process and Automation Engineering
Process Engineering Support of Conceptual Systems, Environmental Monitoring Systems, and Building Automation
Contamination Control Strategy Development
Tech Transfer
One partner, full lifecycle support.

GMP Infrastructure & Systems

Biomanufacturing Readiness

Technical Facilities Management
Related Resources
All Resources

post
Read More

post
Read More

post
Read More
infographic
Read More
Let's move from science to success.
Let’s Talk